In contrast to virtually all other tissues in the body the anatomy of differentiation in the bone marrow remains unknown. This is due to the lack of strategies to examine blood cell production in situ, which are required to better understand differentiation, lineage commitment decisions, and to define how spatial organizing cues inform tissue function.
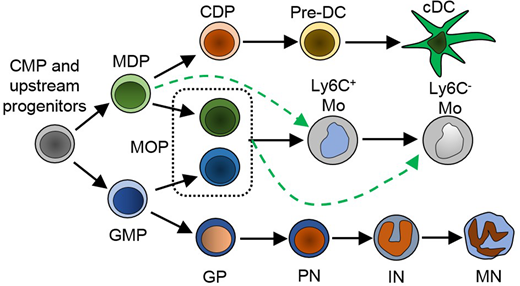
We developed approaches to image and fate map -using confetti mice- myelopoiesis in situ and generated 3D atlases of granulocyte and monocyte/dendritic cell differentiation during homeostasis and after emergency myelopoiesis induced by infection with Listeria monocytogenes. Figure 1 shows stepwise differentiation during myelopoiesis. We have found that -in imaging studies- CD11b-Ly6C-CD117+CD115+ cells are MDP; Lin-CD117+CD16/32+CD115- cells are GMP; CD11b-Ly6C+CD117+CD115+ are MOP; CD11b-CD117+CD115-Ly6C+ are GP; CD11b+CD115+Ly6Chi and CD11b+CD115+Ly6Clo cells are Ly6Chi and Ly6Clo monocytes; and MHCIIhi reticular cells are dendritic cells (DC). We used these markers to map every myeloid cell in the sternum and assessed the relationships between myeloid progenitors, their offspring and candidate niches in situ with single cell resolution. To test whether the interactions observed were specific we obtained the X, Y and Z coordinates for every hematopoietic cell in the sternum (detected using αCD45 and αTer119). We then used these coordinates to randomly place each type of myeloid cell, at the same frequencies found in vivo, through the BM to generate random distributions for each myeloid cell type.
We found that myeloid progenitors do not localize with HSC indicating that they leave the HSC niche during differentiation. In the steady-state GP, MOP, and MDP are found as single cells that do not associate with each other indicating that granulo-, mono-, and dendritic cell-poiesis take place in different location. Myeloid progenitors are specifically recruited to sinusoids but are depleted near endosteum and arterioles (e.g. mean MDP distance to sinusoids, arterioles, and endosteum observed 5, 134, and 105μm vs 9, 86, and 69µm in the random simulation). GP form clusters with preneutrophils and immature neutrophils, in situ fate mapping demonstrated that these clusters are oligoclonal and that additional GP are serially recruited to the cluster as the old ones differentiate. Ly6Clo monocytes and dendritic cells are selectively enriched near MDP (2.0 DC and 4.4 Ly6Clo monocytes observed within 50µm of an MDP vs 0.9 DC and 1.8 Ly6Clo monocytes in the random simulation p=0.02 and p<0.0001). Fate mapping experiments demonstrated that the monocytes around MOP and monocytes and dendritic cells around the MDP are oligoclonal but are not the derived from the MOP/MDP they associate with. These indicate that Ly6Clo monocytes and DC are produced elsewhere but are then selectively recruited to regions enriched in MDP. The results above suggest that different sinusoids might be responsible for supporting different myeloid lineages. We found that dendritic cells localize to a unique subset (8% of all vessels) of colony stimulating factor 1 (CSF1, the major regulator of monopoiesis) -expressing sinusoids. Csf1 deletion in the vasculature disrupted MDP interaction with sinusoids, leading to reduced MDP numbers and differentiation ability, with subsequent loss of Ly6Clo monocytes and dendritic cells. L. monocytogenes infection dramatically changed the architecture of myelopoiesis and caused massive expansion of myeloid progenitors leading to the formation of monoclonal GP clusters and oligoclonal MOP clusters whereas MDP are still found as single cells associated with dendritic cells. Even after this massive insult granulopoiesis and mono/DC poiesis remained spatially segregated to different sinusoids. Csf1 deletion in the vasculature prevented generation of MDP and dendritic cells in response to infection.
In summary we have developed strategies to image and fate map myelopoiesis in situ; revealed spatial segregation of -and distinct clonal architectures for- granulopoiesis and mono/DCpoiesis; and identified rare CSF1+ sinusoids that maintain mono/DCpoiesis in the steady-state and after infection. These data indicate that there is a specific spatial organization of definitive hematopoiesis and that local cues produced by distinct blood vessels are responsible for this organization.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal